Jump To:
- What Are Gas Separation Membranes?
- Gas Separation Membrane Process
- What Are Gas Membranes Made Of?
- Benefits of Using Gas Separation Membranes
- Why Choose Gas Separation Membranes
According to the scientific definition, a membrane is a selectively semipermeable barrier that allows only specific substances to move across it. For example, animal cell membranes allow nutrients to enter the cell while keeping most harmful chemicals out.
Gas separation membranes are a specific type of artificial membrane developed to separate gases for industrial use. This post will explain what gas separation membranes are as well as their common applications and benefits.
What Are Gas Separation Membranes?
Like other membranes, gas separation membranes are semipermeable barriers that only allow specific gases to move across them.
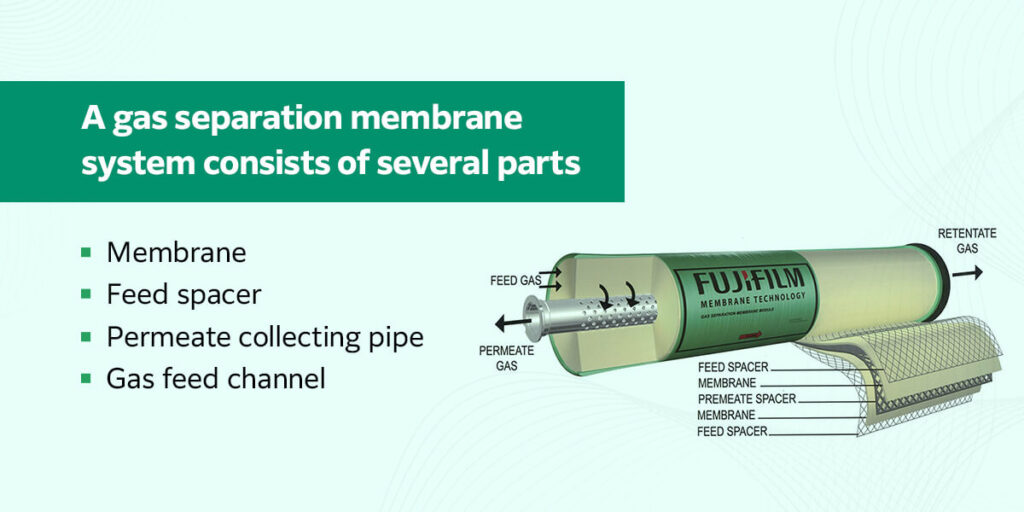
A gas separation membrane system consists of several parts:
- Membrane: This is the polymeric layer that selectively enables certain gases to pass through it. It wraps around the permeate collecting pipe in alternating layers with the feed spacer.
- Feed spacer: This is a layer of netting material placed between the membrane layers to keep the feed channel open and allow particles to move through the system.
- Permeate collecting pipe: This is the pipe that runs through the center of the system and collects the permeate, or the gas that was able to move across the membrane.
- Gas feed channel: This is where the gas enters the system. During the separation process, the feed flow also carries away the retentate, or the molecules that were unable to pass through the membrane.
Each component has a specific solubility and diffusivity, enabling certain materials to pass across the membrane at different rates. Permeability also depends on the materials used to construct the system — the most effective membranes use materials that are both highly selective and highly permeable.
Gas separation membranes have many applications in the industrial space. Some processes that commonly use them include:
- Nitrogen removal from air
- Water (H2O), Hydrogen Sulfide (H2S), and carbon dioxide (CO2) removal from natural gas
- Hydrogen separation
- Volatile Organic Compounds (VOCs) removal from nitrogen or air streams
- Fuel gas conditioning
Gas Separation Membrane Process
In order for the separation process to work, you need a partial pressure gradient — a difference in pressure between each side of the membrane. These two sides are defined as:
- Permeate: The substances that are able to move across the membrane.
- Retentate: The residual substances left over that do not permeate across the membrane.
Because each gas has different properties, it passes across the membrane at a different rate from others, allowing you to effectively separate contaminants. In general, water is one of the fastest-permeating substances while methane is one of the slowest.
Here’s a general explanation of how the process works:
- Compression: The gas enters the compressor and undergoes compression until it reaches the required pressure.
- Pre-treatment: The compressed gas undergoes pre-treatment to make sure no solids and unwanted compounds are carried over to the membrane system. This also includes heating or cooling to the desired inlet temperature;
- Separation: The desired gas components move across the membrane while the rest move on with the retentate.
Unlike in other sweetening methods, such as amine gas treatment, a gas’s molecular structure remains intact throughout the process. The chemicals stay the same, but they become separated.
Gas Diffusion Mechanisms
There are several different ways gases permeate the membrane during the separation phase:
- Material affinity: Some gas species have a tendency to bond with the membrane material, which reduces its ability to quickly permeate the membrane.
- Knudsen diffusion: At low pressures, smaller, lighter molecules are able to move across a membrane faster than heavy ones.
- Solution-diffusion: Gas molecules dissolve when they make contact with the membrane and diffuse through it according to their individual properties.
- Molecular sieving: The size of the membrane’s pores only allows certain molecules to pass through. While this mechanism works well for some substances, it is difficult to design pores small enough to effectively separate gases of similar sizes.
What Are Gas Membranes Made Of?
Although you can use many different porous inorganic materials for gas separation membranes, you’re most likely to find membranes made from dense polymers such as:
- Polysulfone
- Polyamides
- Cellulose derivatives
- Polyaramides
- Polyimides
Polymers are the most common materials for gas separation membranes because they’re economical and can contain thousands of fibers. They also have a high CO2 removal capacity, making them advantageous for natural gas applications such as fuel gas conditioning.
Recent developments in ladder-shaped polymers have allowed researchers to facilitate molecular sieving for molecules that are very similar in size, such as oxygen and nitrogen, without reducing throughput. Although these technologies are still in early development, they could become promising when used at large scale.
Benefits of Using Membrane Gas Separation
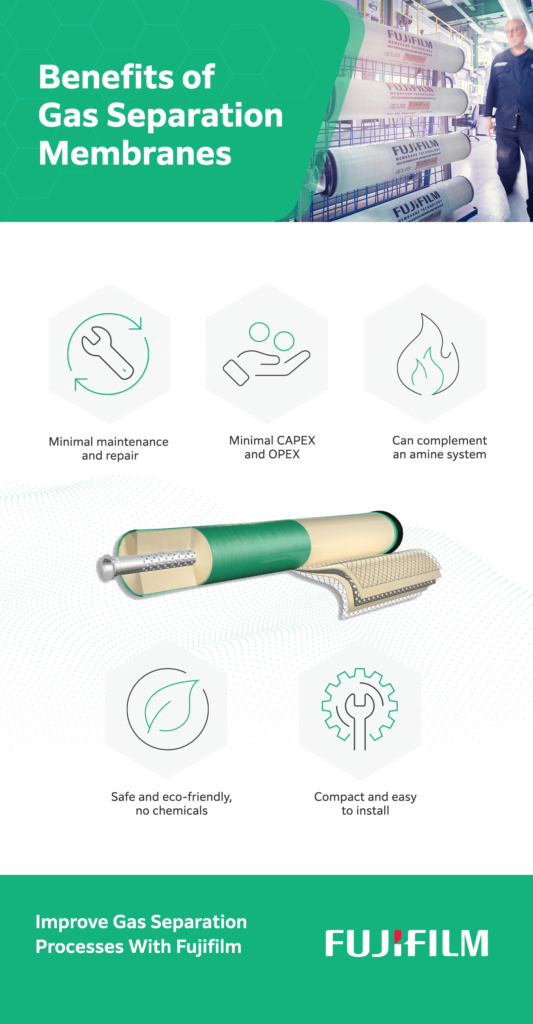
So why use membrane gas separation instead of other treatment methods, like gas conditioning skids or amine gas sweetening? Gas separation membranes offer the following advantages:
- Cost-effective: Operating (OPEX) and capital expenditures (CAPEX) are lower with gas separation membranes than with other gas treatment solutions.
- Energy-efficient: Gas separation using membranes uses significantly less energy than other gas treatment methods, such as amine gas sweetening. This reduces overall utility costs for your facility.
- Simple to use: Thanks to their modular design, gas separation membranes are easy to install and simple to operate, reducing demand for operators and extensive maintenance requirements.
- Environmentally friendly: You can lower your carbon footprint by up to 60% using gas separation membrane technology. Additionally, since the process is free of toxic chemicals and produces lower CO2 emissions, it’s safer for the environment than conventional gas conditioning methods.
- Small footprint: Gas separation membrane units are generally smaller than gas conditioning skids or other similar equipment, making them useful for facilities with limited space.
Why Choose Fujifilm’s Gas Separation Membranes?
If you need gas separation equipment, the innovative Fujifilm Apura™ product line can help you achieve your desired results. The spiral-wound membrane elements allow for selective gas separation and are highly effective for natural gas treatment processes.
We designed the Apura™ – FG membrane specifically for fuel gas conditioning applications with excellent features such as:
- Quick startup and shutdown
- Ability to operate at temperatures of up to 60 degrees Celsius
- Pressure range of up to 83 bars or 8,300 kilopascals (kPa)
- High durability
- Modular design for simple integration into a hybrid system
Our extensive background in organic chemistry means you can count on us to provide the most robust, cost-effective solutions for your gas treatment needs. Whether you need equipment for a new facility or want to upgrade your existing membrane systems, we can provide the knowledge and expertise you need to build an optimal system.
Contact us today or browse our gas membrane white papers for more information.